Emily is a Levonorgestrel releasing Intrauterine System indicated for Intrauterine contraception for up to 5 years and for the treatment of heavy menstrual bleeding.
About EMILY (Levonorgestrel releasing Intrauterine System)
BRIEF PRESCRIBING INFORMATION
This information does not include all details needed to use Emily safely and effectively. See full prescribing information for Emily. Emily (Levonorgestrel releasing Intrauterine System) Approval date (India): 2011 -Full Prescribing Information
INDICATIONS
Emily is a sterile, Levonorgestrel releasing Intrauterine System indicated for:
- Intrauterine contraception for up to 5 years, in women who have at least one child.
- Treatment of heavy menstrual bleeding for women who suffer from dysfunctional uterine bleeding and who are willing to accept LNG IUD as an alternative to hysterectomy or oral medications.
DOSAGE AND ADMINISTRATION
- Release rate of Levonorgestrel is approximately 20 µg per day; Emily should be replaced after 5 years.
- To be inserted by a trained healthcare provider using strict aseptic technique. Healthcare providers are advised to become thoroughly familiar with the insertion instructions before attempting insertion.
- Patient should be re-examined and monitored 4 to 12 weeks after insertion; then, yearly or more often if indicated.
DOSAGE FORMS AND STRENGTHS
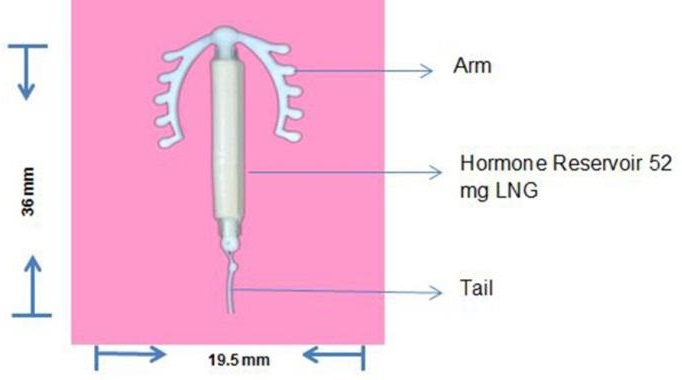
One sterile Intrauterine System consisting of M-shaped polyethylene frame with a steroid reservoir containing 52 mg Levonorgestrel held within an inserter tube.
WARNINGS AND PRECAUTIONS
- If pregnancy occurs with Emily in place, remove Emily.
- Increased risk of ectopic pregnancy
- Group A streptococcal infection has been reported; strict aseptic technique is essential during insertion.
- Before using Emily, consider the risks of PID.
- Bleeding patterns become altered, may remain irregular and amenorrhea may ensue.
- Perforation may occur during insertion. Risk is increased in women with fixed retroverted uteri, during lactation, and postpartum.
- Embedment in the myometrium and partial or complete expulsion may occur.
- Persistent enlarged ovarian follicles should be evaluated
ADVERSE REACTIONS
The most common adverse reactions reported in clinical trials with a similar device (> 10%users) are uterine/vaginal bleeding alterations (51.9%), amenorrhea (23.9%), intermenstrual bleeding and spotting (23.4%), abdominal/pelvic pain (12.8%) and ovarian cysts (12%).
DRUG INTERACTIONS
Drugs or herbal products that induce certain enzymes, such as CYP3A4 may decrease the serum concentration of progestins.
USE IN SPECIFIC POPULATIONS
- Small amounts of progestins pass into breast milk resulting in detectable Steroid levels in infant serum.
- Use of this product before menarche is not indicated.
- Use in women over 65 has not been studied and is not approved.
FAQ
Frequently Asked Questions
- What is Emily (Levenorgestrel Intra uterine system)?
- How does Emily work?
- Is Emily effective in treating Abnormal Uterine Bleeding (AUB)?
- Why should I prefer Emily?
- Is it a difficult process to insert Emily?
- Will my periods change with Emily?
- Which are the conditions were Emily should not be used?
- Are there any potential serious complications and any side effects with Emily?
- How often should I see my doctor once Emily is placed?
- What should I do, if I wish to become pregnant?
- Will my partner be able to feel Emily during intercourse?
- Will Emily protect from HIV or STDs (sexually transmitted diseases)?

Clinical Studies
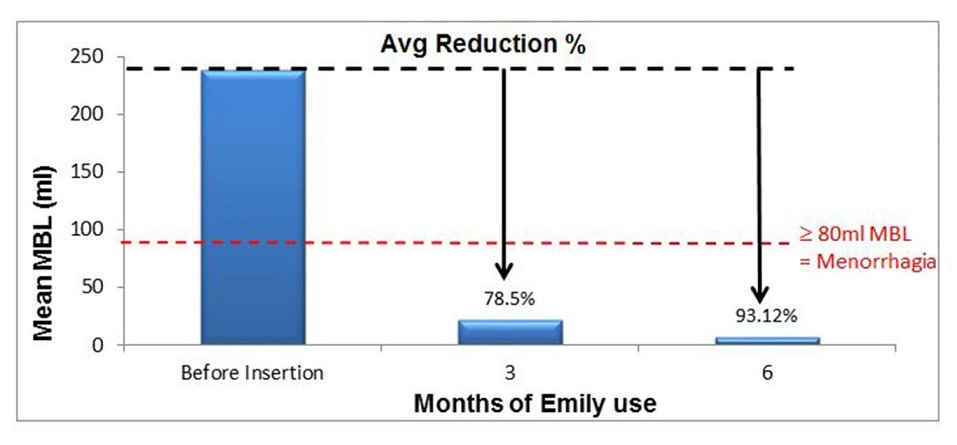
Emily has demonstrated significant clinical benefits in reducing heavy menstrual bleeding (HMB) within 6 months;
The prospective, multicentre, single-arm, phase 4 study involved 45 patients with significant heavy menstrual bleeding. The patients were treated with Emily over a period of 6 months and the patients had shown significant reduction in heavy menstrual bleeding at the end of 6 months. The results are as follows;
Full Publication
Rajmohan Gopimohan , Amrutha Chandran , Joyce Jacob, Sunil Bhaskar , Rajeev Aravindhakshan , Abi S. Aprem. A clinical study assessing the efficacy of a new variant of the levonorgestrel intrauterine system for abnormal uterine bleeding. International Journal of Gynecology and Obstetrics 129 (2015) 114–117. -PDF Version
About R & D
R & D Centre, Thiruvananthapuram, Kerala
Through the interchange of creative, imaginative people, a global Research and Development (R & D) centre that encourages collaboration and cooperation among highly reputed research centres in the country; and a strong commitment to ongoing investment, we have put R & D at the centre of everything we do.
From Blood Transfusion Bags to Hydrocephalus Shunts, once-a-week Non Steroidal Oral Contraceptive Pills and several variants of condoms, every product from HLL is a result of innovation.
The last four decades have seen HLL network with various scientific and academic institutions of excellence for developing novel healthcare products. HLL has a state-of-the-art R & D centre at Thiruvananthapuram in Kerala, India.
 R & D Centre, Thiruvananthapuram, Kerala
R & D Centre, Thiruvananthapuram, Kerala
HLL's R & D centre has several projects in hand, carried out in-house or on collaborative mode, with premier academic and research institutions in the country and abroad. These projects cover a wide area of research ranging from development of novel techniques for drug delivery to blood filters, novel contraceptives and cancer-care devices. Some of the institutions HLL has networked with are-Indian Institute of Technology (IIT), Kanpur; Central Drug Research Institute (CDRI), Lucknow; Sree Chitra Thirunal Institute of Medical Sciences & Technology (SCTIMST), Thiruvananthapuram; Regional Cancer Centre (RCC), Thiruvananthapuram, and Population Council, USA.
HLL has set up a Technology Business Incubation centre (TBlC) at Rajiv Gandhi centre for Biotechnology (RGCB), Thiruvananthapuram. The goal of the TBlC is to develop novel, fast and easy-to-use diagnostic methodologies for various infectious diseases. As part of this collaboration, HLL has developed a duplex kit for viral infections. This project would be extended to development of newer and less expensive diagnostic kits.
Based on its technological competency, the R & D centre is implementing sponsored projects from organisations including Department of Science and Technology (DST), Defense Research Development Organisation (DRDO), Department of Biotechnology (DBT), Council of Scientific Industrial Research (CSlR) and international agencies like Bill & Melinda Gates Foundation.
Uterus defines your Womanhood
Save the Uterus
Save your Womanhood
The status of Indian women has been subjected to many great changes over the past few decades. From equal status with men in ancient times, to equal rights in modern era, history of Indian Women has been really eventful. Today, Women have held high offices in India including President, Prime Minister, to jobs like flying an Aircraft to Business Management and Teaching etc.
But the fate of Indian Women still remains the same when it comes to Healthcare Sector. Despite, several advancements in Healthcare Sector, due to societal cum medical reasons, women are left with no choice but to lose their Womanhood to the hands of Hysterectomy.
Medical reasons for Hysterectomy (Removal of Uterus) vary. Major reason for Hysterectomy is Heavy Menstrual Bleeding, referred in medical terms as Dysfunctional Uterine Bleeding. Almost 40-50% of women in the age group of 40-45 suffer from the problem of heavy bleeding, accompanied with pain and discomfort. This condition is called Heavy Menstrual Bleeding. While most of these cases can be treated by using Medical options, their ineffectiveness in some cases makes the doctors advise Hysterectomy.
In some parts of our country, post completion of the family, uterus is viewed as an unwanted organ. Women approach doctors for uterus removal. While this practice is unwarranted, uterus removal has many complications like increased risk of osteoporosis and heart diseases.
While more and more of Women, post family completion, are opting to remove the uterus to get rid of this indication, Doctors across the Globe have been advocating against the removal of uterus.As per recent data available, every year 40 Lac Indian women undergo Hysterectomy and lose their uterus. While a number of options are available to prevent Hysterectomy, Indian women have been deprived of the same.
“Save the Uterus”, is an initiative by HLL Lifecare Limited (A Govt. of India Enterprise) to educate Indian women about complications involved post Hysterectomy and benefits of preserving their womanhood.
Here are few Frequently Asked Questions, by women who suffer from Heavy Menstrual Bleeding;
- What is Heavy Menstrual Bleeding? How will I know if I am suffering from it?
- Heavy menstrual bleeding is defined as losing more than 80ml of blood over the course of a menstruation cycle.
- You may be suffering from HMB if you are using more number of sanitary pads than normal, pass blood clots or are flooding with sudden heavy loss.
- What are the Treatment options available?
- Treatment includes both medical and surgical options:
- Medical options include Tranexamic acid, Oral Contraceptive Pills, Ormeloxifene and Hormonal Intra-Uterine Systems (Levonorgestrel based).
- Surgical options include Hysterectomy and other endometrial procedures.
- I am already 45 and done with my family planning; shall I get my uterus removed as a permanent solution?
- Many women feel that hysterectomy is a permanent solution while it may be a door to many other serious consequences.
- Always refer your doctor for a second opinion and make sure that there is sufficient indication for hysterectomy.
- What are the consequences of Hysterectomy?
- Injuries: to ureter or bladder bowel
- Hemorrhage: one of the most serious post-operative complication
- Infection: 4% to 25% patients suffer from it after hysterectomy
- Urinary incontinence, high risk of osteoporosis & heart diseases
- Pain, hot flashes, vaginal dryness, weight gain, depression, decreased libido
- My doctor has prescribed me some medicine, but still I am suffering from Heavy Menstrual Bleeding. Should I undergo Hysterectomy?
- Medicines are prescribed by doctor as a first line of treatment in Management of Heavy Menstrual Bleeding. However, if these medications fail to provide you relief, Hormonal Intra-Uterine Systems (like EMILY) are suggested to treat to Indication.
- IUS are one of the most effective and economical alternative to hysterectomy in most cases of Heavy Menstrual Bleeding.
- With marked reduction in Blood loss, they improve the quality of life by reducing the pain associated with bleeding.
- Studies suggest that 70% of hysterectomies cases can be prevented using Hormonal-Intra Uterine Systems
- I want to “Save my Uterus”. How will I get a Hormonal Intra-Uterine System?
- Hormonal Intra-Uterine systems are devices which are inserted in body by a doctor only.
- For insertion of an IUS, please refer to your doctor for Hormonal IUS (EMILY)
- For more information on Hormonal Intra-Uterine Systems, please visit www.emily.org.in
Our contact information
We’re open to talk to nice people
Call us from 10 am to 7 pm
I want Emily
HLL Lifecare Limited, Akkulam, Trivandrum Kerala - 695017
Just say hello
Open the map
Close the map







 EMILY CLINICAL TRIAL PAPER
EMILY CLINICAL TRIAL PAPER